 INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
Sodium hyaluronate from fermentation 2.0 %. Viscoelastic solution for use into the joint cavity. Sterile by moist heat.
COMPOSITION:
1 ml isotonic solution (pH 7.3) contains 20.0 mg sodium hyaluronate from fermentation and sodium chloride, sodium monohydrogenphosphate, sodium dihydrogenphosphate, mannitol and water for use.
INDICATIONS:
Pain and restricted mobility in degenerative and traumatic changes of the knee joint and other synovial joints.
CONTRA-INDICATIONS:
OSTENIL® Plus should not be used in patients with ascertained hypersensitivity to one of
the constituents.
PRECAUTIONS:
Caution should be exercised in patients with known hypersensitivity to drugs. The general precautions for intraarticular doses should be observed, including measures to avoid joint infections. OSTENIL Plus should be delivered accurately into the joint cavity, if necessary under imaging control. Avoid doses into blood vessels or surrounding tissues! As no clinical evidence is available on the use of hyaluronic acid in children, pregnant and lactating women or in inflammatory joint diseases such as rheumatoid arthritis or Bechterew disease, treatment with OSTENIL Plus is not recommended in these cases. Do not use if the pre-filled disposable or sterile pack are damaged. Store between 2 °C and 25 °C! Do not use after the expiry date indicated on the box. Keep out of the reach of children.
SIDE EFFECTS:
Local secondary phenomena such as pain, feeling of heat, redness and swelling may occur in the joint treated with OSTENIL Plus. Application of an ice pack for five to ten minutes onto the treated joint will reduce the incidence of these events.
INTERACTIONS WITH OTHER PRODUCTS:
No information on the incompatibility of OSTENIL Plus with other solutions for intra-articular use is available to date. The concomitant use of an oral analgesic or anti-inflammatory drug
during the first few days of treatment may be helpful for the patient.
DOSAGE AND ADMINISTRATION:
Use OSTENIL Plus into the affected joint once a week for a total of 1-3 doses. Several joints may be treated at the same time. Repeat treatment cycles may be administered as required. In case of joint effusion it is advisable to reduce the effusion by aspiration, rest, application of an ice pack and/or intra-articular corticosteroid use. Treatment with OSTENIL Plus can be started two to three days later. The content and the outer surface of the OSTENIL Plus prefilled disposable are sterile as long as the sterile pack remains unbroken. Take the pre-filled disposable out of the sterile pack. Before usage of the pre-filled disposable the tamper-evident seal has to be tilted up. As a result the crosspieces of the tamperevident seal break and the cap can be removed together with the tip cap (see pictures). Attach a suitable needle (for example 18 to 25 G) and secure it by turning slightly. If present remove the air bubble from the disposable prior to use.
CHARACTERISTICS AND MODE OF ACTION:
Synovial fluid, which is viscoelastic due to the presence of hyaluronic acid, is found in all synovial joints, particularly the large weight bearing joints, where it ensures normal, painless movement due to its lubricating and shock-absorbing properties. It is also responsible for the nutrition of the cartilage. In degenerative joint disorders such as OA, the viscoelasticity of the synovial fluid is markedly reduced thereby decreasing its lubricating and shock-absorbing functions. This increases mechanical loading of the joint and cartilage destruction which ultimately results in pain and restricted mobility of the affected joint.
Supplementing this synovial fluid with intra-articular doses of highly purified hyaluronic acid can ameliorate the viscoelastic properties of synovial fluid. This improves its lubricating and shock-absorbing functions and reduces mechanical overload of the joint. As a rule this results in a decrease in pain and an improvement in joint mobility which may last for several months after a treatment cycle. OSTENIL Plus is a transparent solution of natural and highly purified sodium hyaluronate obtained by fermentation and is devoid of animal protein. OSTENIL Plus also contains mannitol, a free radical scavenger, which helps to stabilise the chains of sodium hyaluronate. In biocompatibility studies, OSTENIL Plus was found to be particularly safe.
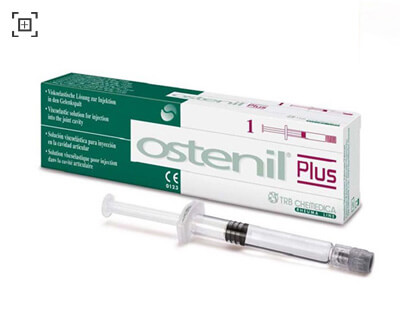
PRESENTATION AND PACKAGE SIZE:
One pre-filled disposable of 40 mg/2.0 ml OSTENIL Plus in a
sterile pack.
 INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE