 INTENDED USE:
INTENDED USE:
Viscoelastic solution for use into the joint cavity.
COMPOSITION:
- Active substance
Sodium hyaluronate
- Excipients
Sodium chloride, sodium monohydrogenphosphate, sodium dihydrogenphosphate, water for use.
INDICATIONS:
Pain and restricted mobility in degenerative and traumatic changes of the knee joint and other synovial joints.
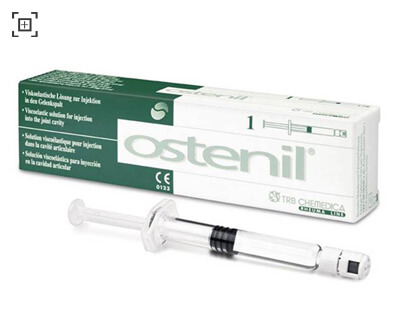
PRESENTATION:
Sterile pre-filled disposable containing 20mg/2ml sodium hyaluronate as an isotonic solution, in a sterile pack.
DOSAGE & ADMINISTRATION:
Depending on the size of the joint use 2ml or less of OSTENIL into the joint cavity once a week for 3 to 5 doses. Several joints may be treated at the same time. The beneficial effects of a treatment cycle of five intra-articular doses may last for several months depending on the severity of the joint changes. Repeated treatment cycles may be administered as required. In case of joint effusion it is advisable to reduce the effusion by aspiration, rest, application of an ice pack and/or intra-articular
corticosteroid use. Treatment with OSTENIL can be started two to three days later.
The contents and outer surface of the OSTENIL pre-filled disposable are sterile as long as the sterile pack remains unbroken. Take the pre-filled disposable out of the sterile pack, remove the Luer lock cap from the disposable, attach a sterile cannula (19 to 21 G) and secure it by turning slightly. Remove the air bubble from the disposable prior to use.
CHARACTERISTICS AND MODE OF ACTION:
OSTENIL contains a highly purified specific fraction of sodium hyaluronate produced by fermentation from bacteria. The molecular weight range of OSTENIL is designed to achieve optimum activity with additional benefits ancillary to the mechanical action and primarily related to the viscoelastic characteristics. The product is free from animal proteins and the narrow molecular weight range helps to ensure the absence of low weight pro-inflammatory fragments.
Hyaluronic acid (HA) is a natural polymer, which provides viscoelastic properties to the synovial fluid. In a normal joint it is highly concentrated at the surface coating of articular cartilage as well as the superficial layers of the synovial membrane. In the synovial fluid HA acts as a lubricant and a shock absorber, an energy storing agent between opposing cartilages, a semi-permeable barrier regulating metabolic exchanges between the cartilage and the synovial fluid, a cell traffic controlling agent and a viscoelastic shield around the synoviocytes and adjacent nerve endings. In degenerative joint disorders such as OA the synovial HA is fragmented and depolymerised with a corresponding reduction in viscoelasticity. This increases the mechanical loading of the joint and results in cartilage breakdown and therefore pain and restricted mobility of the affected joint.
A treatment cycle with OSTENIL helps restore the synovial homeostasis of the affected joint, thereby improving the viscoelastic properties of the synovial fluid and restoring the lubricating and shock absorbing functions. The net result is a decrease in pain and an improvement in joint mobility which
may last for several months after the end of the treatment cycle.
BIOCOMPATIBILITY:
Results of acute, sub-acute and chronic toxicity studies together with the results of the foetal toxicity, fertility, peri- and post-natal toxicity studies show that sodium hyaluronate is well tolerated.
INTERACTIONS:
No information on the incompatibility of OSTENIL with other solutions for intra-articular use is available to date.
STORAGE:
Store at 25°C in original sterile pack, away from heat sources and light. Do not freeze.
SHELF-LIFE:
3 years if stored in original unopened package at room
temperature i.e. 25°C.
 INTENDED USE:
INTENDED USE: